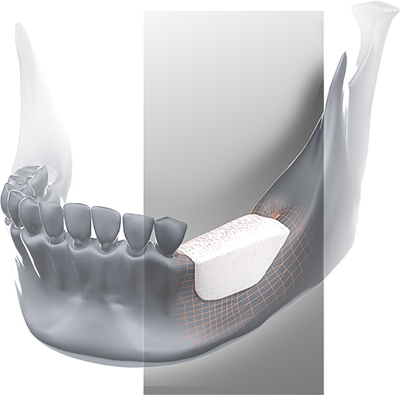
BIOBank Synergy® is a 100% natural DBM* putty, the result of over 10 years of R&D and a globally patented manufacturing process.
*Demineralized Bone Matrix with 25% of residual minerals
From bone powder…to BIOBank Synergy®!
This product is made from our allogeneic bone powders, processed using our exclusive Supercrit® method. An additional step completes the process, resulting in a unique putty that retains both collagen and minerals to promote optimal bone regeneration.
Advantages

The range
BIOBank Synergy® is available in two volumes and packaged individually in a syringe in double sterile packaging.
| Reference | Name |
| 800105 | BIOBank Synergy® 0,5 cc |
| 800110 | BIOBank Synergy® 1 cc |
Indications
BIOBank Synergy® is indicated for filling bone defects in maxillofacial, oral and dental surgery in the following cases:
- Peri-implant defects
- Alveolar preservation
- Sinus lifts
- Osteotomy filling
Traceability of allogeneic grafts
Each product package contains the following sheets :
- Graft identity sheet >> to be filed in the patient’s medical record
- Implant sheet >> to be sent duly completed and signed to BIOBank
DOCUMENTATION
► Download the BIOBank Synergy® brochure
Bibliographic references
Fages et al. (1994) Use of Supercritical CO2 for Bone Delipidation. Biomaterials, 15(9):650-656.
Vastel et al. (2008) Comparative Ultrasound Evaluation of Human Trabecular Bone Graft Properties After Treatment with. Different Sterilization Procedures. J Biomed Mater Res B Appl Biomater, 90(1):430-7.
Tournier et al. (2021) A partially demineralized allogeneic bone graft: in vitro osteogenic potential and preclinical evaluation in two different intramembranous bone healing models. Scientific Reports, 11:4907
Fages et al. (1998) Bone Allografts and Supercritical Processing: Effects on Osteointegration and Viral Safety. J of Supercritical Fluids, 13:351-356.